Draw The Electron Configuration For A Neutral Atom Of Manganese
Draw The Electron Configuration For A Neutral Atom Of Manganese - The shorthand electron configuration (or noble gas configuration) as. The electron configuration of manganese, atomic number 25, is: Web atom manganese has 25 25 25 electrons. The atomic number of cl is 17. Web a) write the complete electron configuration for a neutral manganese atom and indicate the number of valence electrons contained in this atom. To write the configuration for the manganese. You can easily understand the derivation. Web how to write electron configurations and orbital diagrams (general chemistry i) med science streamlined. An atom has a valence shell electron. A neutral chlorine atom has 17. For determining its electron configuration, we first lo. B) give a valid set of quantum. The atomic number of cl is 17. Web using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral chlorine atom. The atomic number of cl is 17. To write the configuration for the manganese. Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. Write the atomic orbital diagram for the $4 s$ and $3 d$ electrons in a(a) manganese atom. #1s^2, 2 s^2,. Number next to the letters 's', 'p', 'd' or 'f'. Web today in this video, we will help you determine the electron configuration for the manganese element. To write the configuration for the manganese. Web how to write electron configurations and orbital diagrams (general chemistry i) med science streamlined. Web a) write the complete electron configuration for a neutral manganese. An atom has a valence shell electron. To properly fill the electron configuration chart follow the instructions below given in a figure. B) give a valid set of quantum. Web an electrically neutral atom has the following electron configuration: Web using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral chlorine atom. What is the name of this atom? Number next to the letters 's', 'p', 'd' or 'f'. A neutral chlorine atom has 17. Web today in this video, we will help you determine the electron configuration for the manganese element. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features. An atom has a valence shell electron. For determining its electron configuration, we first lo. Web a) write the complete electron configuration for a neutral manganese atom and indicate the number of valence electrons contained in this atom. Web draw the electron configuration for a neutral atom of manganese energy this problem has been solved! A neutral chlorine atom has. Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. B) give a valid set of quantum. Web an electrically neutral atom has the following electron configuration: Web draw the electron configuration for a neutral atom of. B) give a valid set of quantum. Electron configuration chart of all elements is mentioned in the table below. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Write the atomic orbital diagram for the $4 s$ and $3 d$ electrons in a(a) manganese atom. For determining its electron configuration, we first lo. Web how to write electron configurations and orbital diagrams (general chemistry i) med science streamlined. Write the atomic orbital diagram for the $4 s$ and $3 d$ electrons in a(a) manganese atom. Web the atomic number of manganese (mn) is 25. The electron configuration of manganese, atomic number 25, is: An atom which does not contain any charge and there. A neutral chlorine atom has 17. What is the name of this atom? Web atom manganese has 25 25 25 electrons. Web using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral chlorine atom. The atomic number of cl is 17. Web using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral chlorine atom. Web a) write the complete electron configuration for a neutral manganese atom and indicate the number of valence electrons contained in this atom. Number next to the letters 's', 'p', 'd' or 'f'. Web draw the electron configuration for a neutral atom of manganese energy this problem has been solved! An atom has a valence shell electron. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. For determining its electron configuration, we first lo. A neutral chlorine atom has 17. An atom which does not contain any charge and there is no loss of electrons or gain of electrons is called a neutral atom. B) give a valid set of quantum. The electron configuration of manganese, atomic number 25, is: Web the atomic number of manganese (mn) is 25. To write the configuration for the manganese. Web today in this video, we will help you determine the electron configuration for the manganese element. You can easily understand the derivation. Web how to write electron configurations and orbital diagrams (general chemistry i) med science streamlined.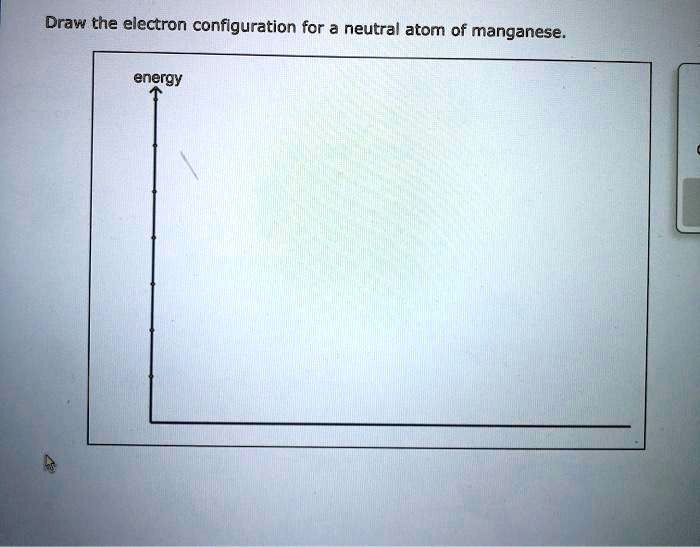
SOLVED Draw the electron configuration for a neutral atom of manganese

WebElements Periodic Table » Manganese » properties of free atoms

WebElements Periodic Table » Manganese » properties of free atoms

Electron Configuration For Manganese Atomic Number 25 How Do You Draw
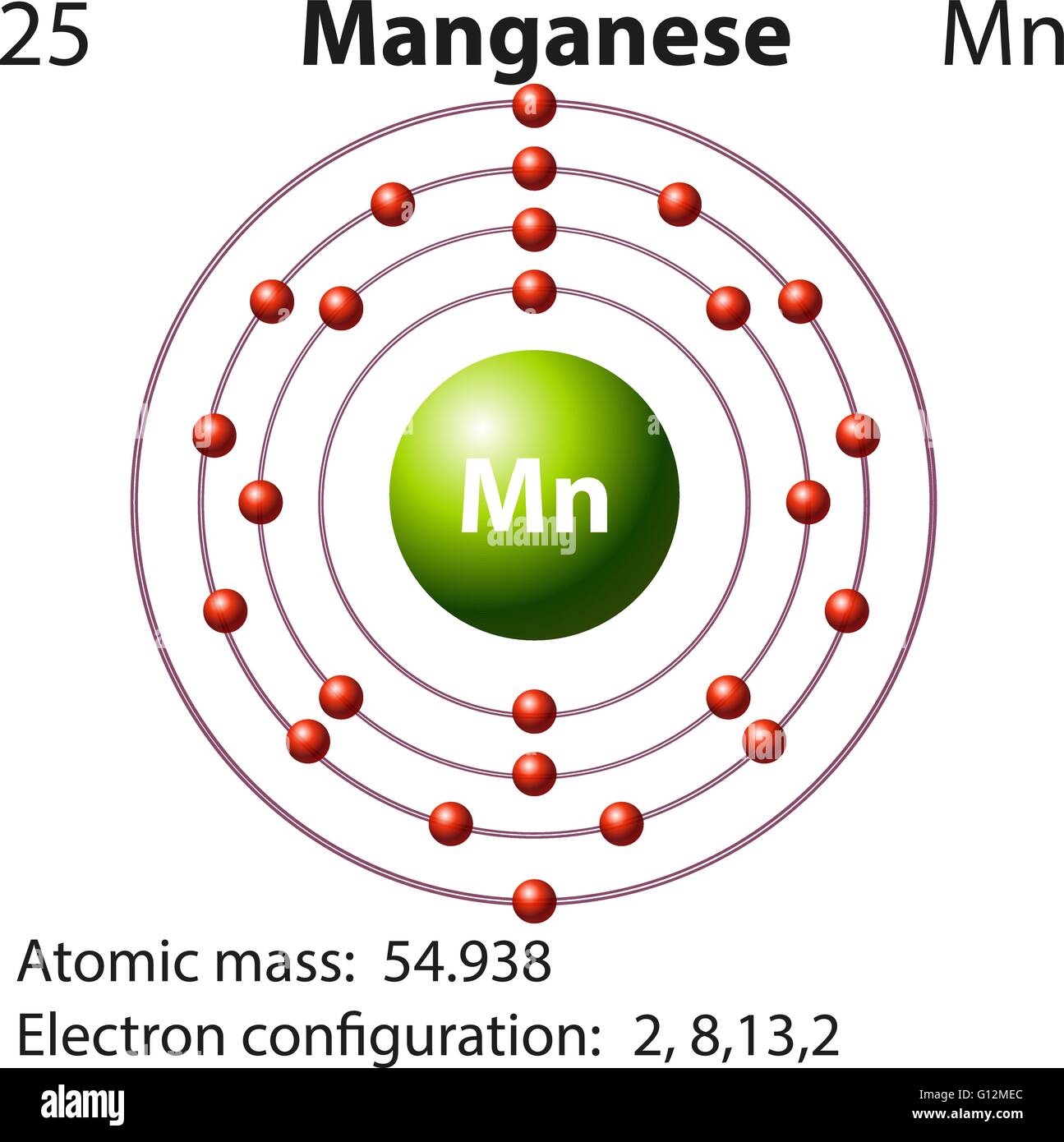
Symbol and electron diagram for Manganese illustration Stock Vector

Electron Configuration of Manganesse Mn Lesson YouTube
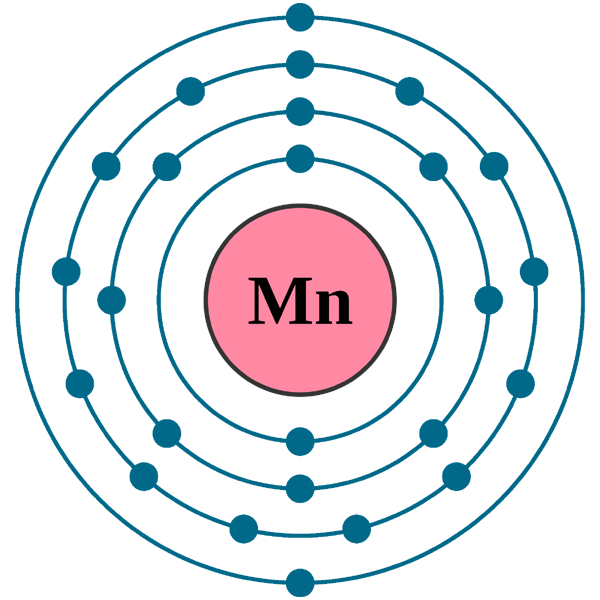
Manganese electron configuration Newton Desk
Manganese Electron Configuration Manganese Orbital Diagram Insight
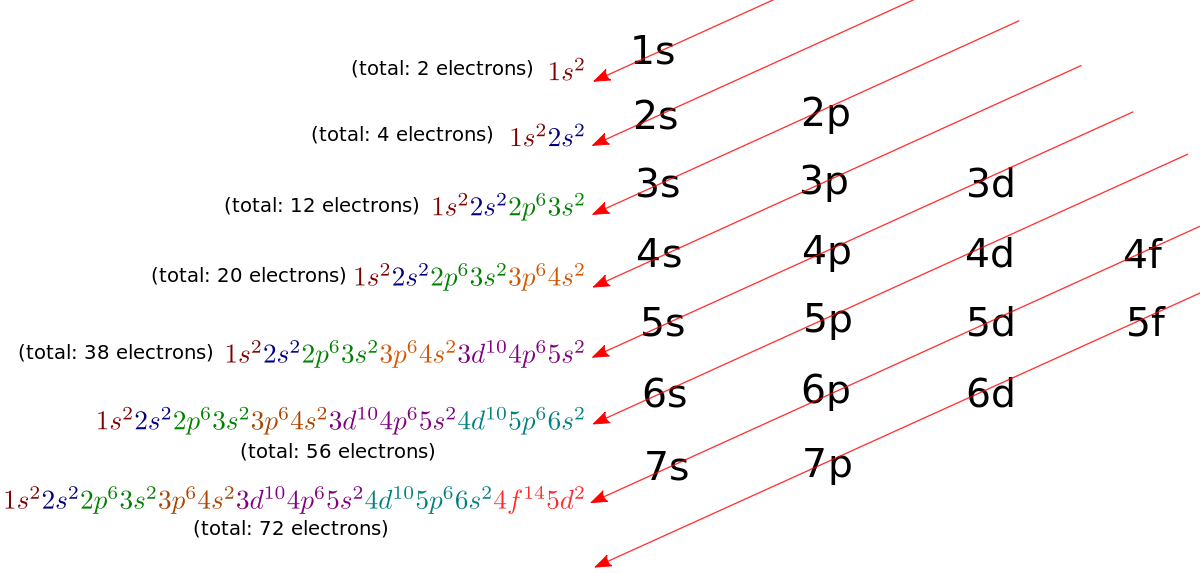
Electron Configuration For Manganese Atomic Number 25 Manganese
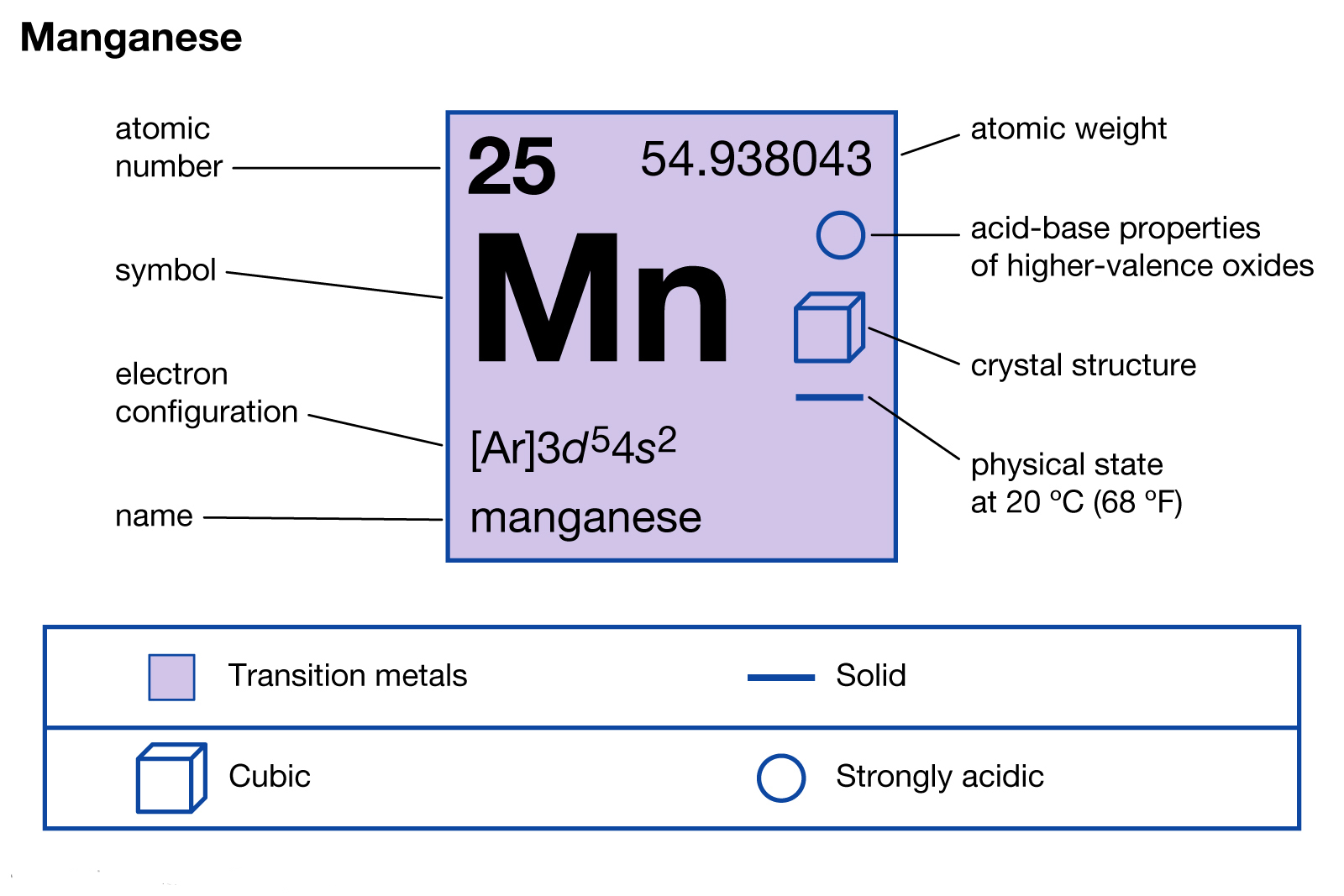
Manganese Electron Configuration Dynamic Periodic Table of Elements
Web Atom Manganese Has 25 25 25 Electrons.
A Neutral Chlorine Atom Has 17.
Write The Atomic Orbital Diagram For The $4 S$ And $3 D$ Electrons In A(A) Manganese Atom.
#1S^2, 2 S^2, 2P^6, 3S^2, 3P^4#.
Related Post: